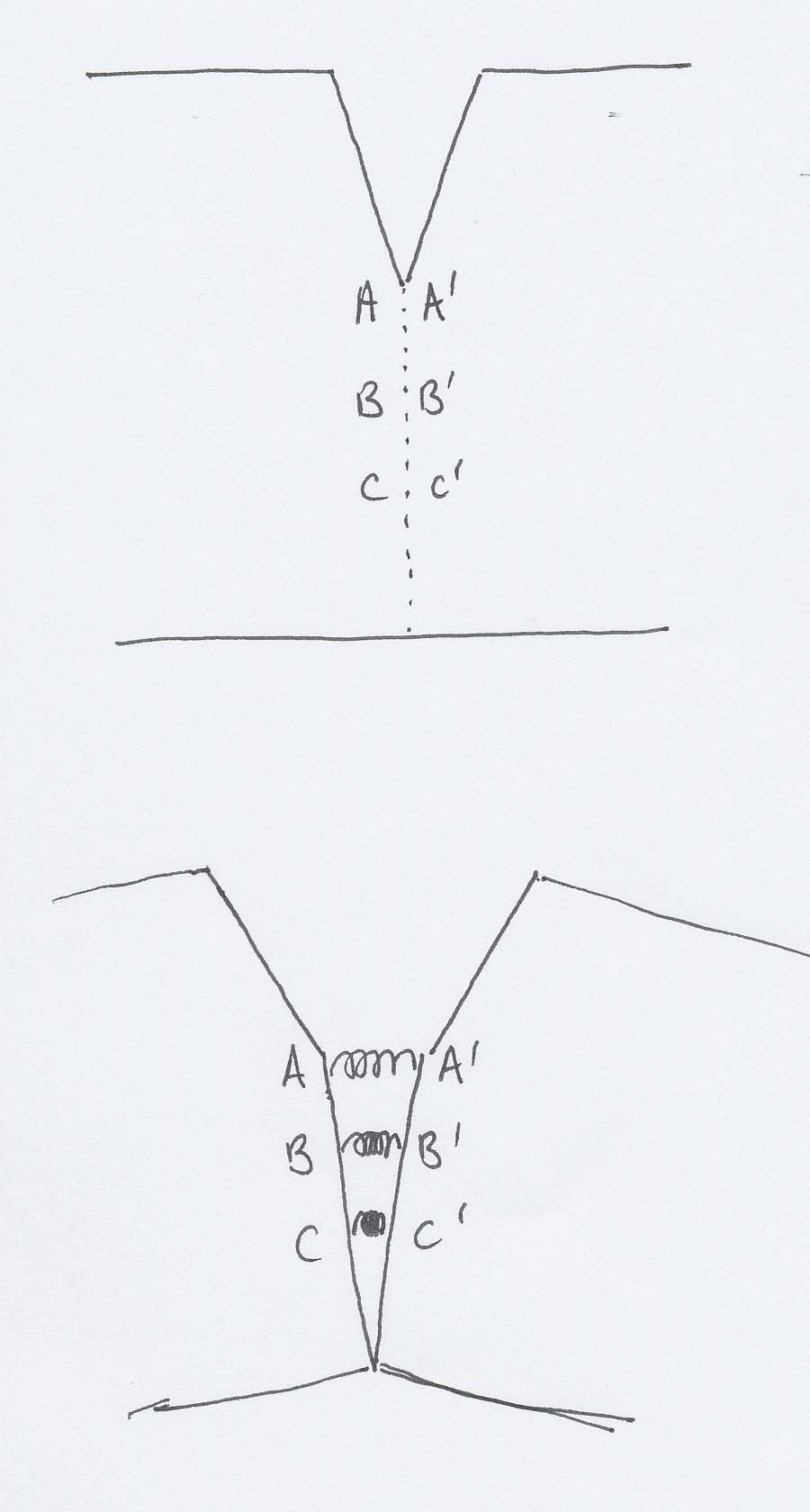
Imagine a specimen with a crack already present. We will bend it, and consider what happens to the pairs of atoms AA', BB' and CC'.
If we represent the relevant intermolecular forces by springs, and imagine the lines of atoms each side of the dotted line to open out as in the lower diagram, then you will see that A and A' will end up further apart than C and C'. Since tension is proportional to separation for springs (and for atoms), there is a greater tension between A and A' than between any other pair of atoms down the line, so the stress is concentrated there. The total of all the tensions is what resists our attempt to bend the specimen, and holds it in equilibrium.
If we exert a greater bending moment, we increase the intermolecular forces on all the atoms, but most of all on A and A'. These two reach the maximum on the Force-separation curve before any of the others, by which time there is about half an atomic diameter of clear space between A and A'. At this point one of two things happens.
-
If the atoms cannot adjust their positions to relieve the stress, A and A' come apart. The situation now is that the bending moment we are applying is being resisted by one fewer pairs of atoms, so they are all taking a slightly greater share. B and B' are already taking the As' place as chief load bearer, and they have an even greater cross to bear. So they come apart, too. So it goes on, and the material collapses along this line.
-
If, on the other hand, plastic deformation is possible, the atoms can all slide around, and some will so slide as to fill in the space between A and A'. This removes the stress, and the specimen will be permanently bent. But whole.
Start from the notion that the ions are held in a lattice, without worrying about how this is achieved. Permeating this is a 'sea' of electrons. The reason it is a 'sea' is that the electrons in question (generally the electrons in the outermost shell) would normally sit at a particular distance from their parent atom, but the ionic centres are closer together than this: which means that an electron between two ions is in a no-mans-land, belonging to both ions. So the electron can swim around all the ions freely (it can't go through the middle of them of course!), following the course of a coracle floating on a river meandering its way around lots of little hillocks.
In an insulator the atoms are just that bit further apart (insulators tend to be less dense than conductors), and the outer electrons are held more firmly to their atoms, while the atoms are held less firmly to each other. However, if you could excite an outer electron into the next energy level up, because higher energy levels usually have higher radii associated with them, the excited electron would now be able to drift into the next-door atom's territory, and so would be able to drift around the place. For most insulators the exciting energy required to do this is so high that the material would melt before you had achieved it.
A semiconductor is a material in which the energy required to promote a valence electron into the conduction band is quite low, so at room temperatures a small number of electrons are able to drift around at this higher level. Warming such a material promotes more electrons into this level, and the conductivity increases (conductivity µ number of free electrons per unit volume (among other things))
Resistance arises when electrons drifting along (a current) arrive at a dislocation, because then the next-door atom isn't sufficiently close to be jumped to after all. So the electron comes crashing to a halt, turns round and goes off in another direction so as to avoid the dislocation. This process imparts energy to the region around the dislocation, and the ions around the collision vibrate more vigorously. Hence the electron has been slowed in its progress and its energy has been transferred to the material as thermal energy.
If you heat a material, the extra random lattice vibrations mean that temporary 'dislocations' keep coming into being that impede electron flow: thus the rise of resistance in hot materials. In a semiconductor, the promotion of electrons into the conduction band is an even more important effect, which is why thermistors (lumps of semiconductor) have negative temperature coefficients of resistance.
Furthermore, the increased vibration of the dislocations means that any electrons making contact are bounced away with more energy than they had before (super-elastic collisions). Because the electrons form a sea (or gas, as it is often perceived in this context), they are not confined, and shoot off to quite distant parts of the material carrying their newly acquired KE with them. They then interact with dislocation in the new place, having by-passed all the intervening ions, dumping their energy, thereby effecting a transfer of thermal energy from the part being heated to a cooler part. That is why good conductors of electricity tend to be good conductors of thermal energy (The Wiedemann-Franz Law). Insulators have to transfer thermal energy by the much less efficient process of bumping into the next door atom, and passing it down the line, one atom at a time.